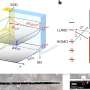
Synthesis and application of the new reagent Ph3PCN2. Credit: Max M. Hansmann
Professor Max Martin Hansmann from the Department of Chemistry and Chemical Biology and his team have developed a new reagent for selectively adding carbon atoms to molecules. This promising development for basic research in organic chemistry has been published in Science.
The research work was conducted as part of the ERC Starting Grant awarded to Professor Hansmann in 2022. Dr. Taichi Koike, postdoctoral researcher and the first author of the paper, is working as a Alexander von Humboldt Research Fellow at the Chair of Organic Chemistry headed by Professor Hansmann.
"The precise modification of molecules at a single-atom level is one of the most elegant transformations in organic chemistry," says Professor Hansmann. It is interesting for researchers because it has the potential to allow access to complex pharmaceuticals in a shorter synthetic sequence. However, the development of reagents that can selectively introduce a carbon atom is a challenging task.
Professor Max Hansmann and his team have now succeeded in synthesizing a reagent that can serve not only as a carbon atom source but also as a multipurpose transfer reagent.
"We are confident that further exploration of the reactivity of this type of reagent will facilitate new applications in the transfer of carbon atoms, for instance as a new reagent in accessing higher cumulenes or in the late-stage functionalization of complex molecular structures," says Professor Hansmann.
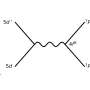
To develop the reagent, the team used a simple approach for stabilizing a carbon atom—the coordination with two neutral electron-donating groups. The resulting species, known as carbones (L1→C←L2), had hardly been explored to date as carbon atom sources.
The challenges involved in using carbones as sources of carbon atoms led the researchers to develop a reagent in which a carbon atom is flanked by two simple and labile groups (PPh3 and N2).
In their paper, they describe the synthesis of the crystalline and isolable reagent Ph3P=C=N2 by a formal PPh3/N2 exchange reaction using carbodiphosphorane Ph3P=C=PPh3 and nitrous oxide (N2O).
This synthetic approach is elegant and very simple because no azides are required, which is typically the case with diazo compounds and constitutes a safety risk in the synthesis. Professor Hansmann and his team were able to demonstrate that this reagent serves as a highly selective transfer reagent for fragments from the carbon atom without requiring any further additives.
Ph3PC transfers to ambiphiles result in phosphorus ylide-terminated heterocumulenes, CN2 transfers to alkenes in multi-substituted pyrazoles. Ultimately, in the reaction with carbonyl compounds, a carbon atom transfer takes place, which leads to either various alkynes or butatrienes.
More information: Taichi Koike et al, Ph 3 PCN 2 : A stable reagent for carbon-atom transfer, Science (2024). DOI: 10.1126/science.ado4564
Journal information: Science
Provided by TU Dortmund University