Learn more: PMC Disclaimer | PMC Copyright Notice
High altitude pulmonary edema-clinical features, pathophysiology, prevention and treatment
Abstract
High altitude pulmonary edema (HAPE) is a noncardiogenic pulmonary edema which typically occurs in lowlanders who ascend rapidly to altitudes greater than 2500-3000 m. Early symptoms of HAPE include a nonproductive cough, dyspnoea on exertion and reduced exercise performance. Later, dyspnoea occurs at rest. Clinical features are cyanosis, tachycardia, tachypnoea and elevated body temperature generally not exceeding 38.5°C. Rales are discrete initially and located over the middle lung fields. HAPE mainly occurs due to exaggerated hypoxic pulmonary vasoconstriction and elevated pulmonary artery pressure. It has been observed that HAPE is a high permeability type of edema occurring also due to leaks in the capillary wall (‘stress failure’). Slow descent is the most effective method for prevention; in addition, graded ascent and time for acclimatization, low sleeping altitudes, avoidance of alcohol and sleeping pills, and avoidance of exercise are the key to preventing HAPE. Treatment of HAPE consists of immediate improvement of oxygenation either by supplemental oxygen, hyperbaric treatment, or by rapid descent.
INTRODUCTION
Sojourns to high altitude are common for adventure and recreational purposes. Too rapid an ascent or inability to acclimatize leads to high altitude illnesses. These include acute mountain sickness (AMS), high altitude cerebral edema (HACE) and high altitude pulmonary edema (HAPE).
The most common cause of death related to high altitude, HAPE is completely and easily reversed if recognized early and treated properly. HAPE is a non-cardiogenic pulmonary edema which occurs in two forms. The first form typically occurs in un-acclimatized lowlanders who ascend rapidly to altitudes greater than 2500-3000 m. The second form, also called re-entry HAPE, occurs in high landers returning after a sojourn at a lower altitude. The two forms very probably share the same pathophysiology. This article discusses the pathophysiological mechanisms responsible for HAPE and elaborates on various modalities for its prevention and treatment.
HISTORY OF HIGH ALTITUDE PULMONARY EDEMA
HAPE was misdiagnosed for centuries, as evidenced by frequent reports of young, vigorous men suddenly dying of “pneumonia” within days of arriving at high altitude. The death of Dr. Jacottet, “a robust, broad-shouldered young man,” on Mont Blanc in 1891 (he refused descent so that he could “observe the acclimatization process” in himself) may have provided the first autopsy report of HAPE. Angelo Mosso wrote, “From Dr. Wizard's post-mortem examination.… the most immediate cause of death was therefore probably a suffocative catarrh accompanied by acute edema of the lungs.… I have gone into particulars of this sorrowful incident because a case of inflammation of the lungs also occurred during our expedition, on the summit of Monte Rossa, from which, however, the sufferer fortunately recovered.”
On an expedition to K2 (Karakoram Range, Pakistan) in 1902, Alistair Crowley described a climber “suffering from edema of both the lungs and his mind was gone.”[1] In the Andes, physicians were familiar with pulmonary edema peculiar to high altitude, but the English speaking world was largely oblivious to the phenomenon. This changed in 1960 when Charles Houston, an Aspen internist, reported on a healthy 21-year old cross country skier who developed pulmonary edema while crossing a 3,650-meter pass. His chest radiograph showed pulmonary edema, while his electrocardiogram displayed nonspecific changes. Although such cases had been termed “high altitude pneumonia”, Houston recognized this to be acute pulmonary edema without heart disease. Houston's series of four patients included a 26-year old physician who undertook a self-examination with a sthethoscope and could detect fine, moist rales.[2] Hultgren performed cardiac catheterization on seven patients with HAPE at the Chulec General Hospital in Peru. The patients had pulmonary hypertension, reduced cardiac outputs, and low pulmonary artery occlusion pressure.[3] The large of number of Indian troops stationed in the Himalayas provided further for description of HAPE in otherwise young healthy men.[4,5] Since then, many studies and reviews have been published and HAPE is still the subject of intense investigation.
EPIDEMIOLOGY
The estimated incidence of HAPE in visitors to ski resorts in the Rocky Mountains of Colorado is 0.01-0.1%.[6] In a general alpine mountaineering population, the prevalence of HAPE is <0.2%.[7] The HAPE incidence among trekkers in the Himalayas and climbers in the Alps ascending at a rate > 600m/day is around 4%.[8,9] In an unselected population of Indian soldiers, airlift to an altitude of 5500m was associated with a HAPE incidence of up to 15%.[10]
CLINICAL FEATURES
HAPE presents within 2-5 days of arrival at high altitude. It is rarely observed below altitudes of 2500-3000 m and after 1 week of acclimatsation at a particular altitude.
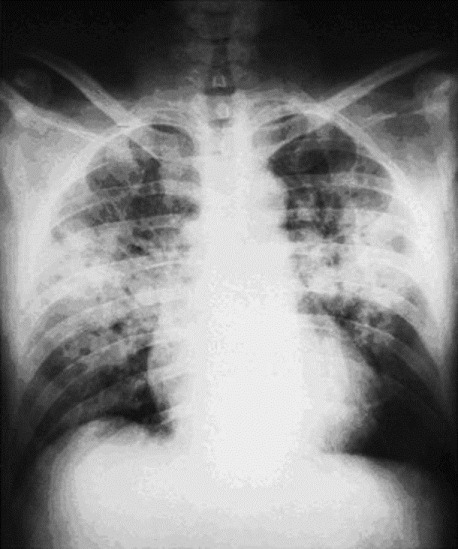
In most cases, it is preceded by symptoms of acute mountain sickness. Early symptoms of HAPE include a subtle nonproductive cough, dyspnoea on exertion and reduced exercise performance. As HAPE progresses, cough worsens and the subject may have a debilitating degree of dyspnoea, even at rest. Orthopnea may occur. Gurgling in the chest and pink frothy sputum indicate advanced cases. The clinical features are cyanosis, tachypnoea, tachycardia and elevated body temperature generally not exceeding 38.5°C. Rales are discrete initially and located over the middle lung fields. Imaging of the thorax reveals patchy opacities with inconsistent predominance of location, but often infiltrates are seen in the region of the right middle lobe [Figure 1].[11,12]
Subclinical HAPE probably occurs and causes no or minimal symptoms which can be ignored or attributed to other factors. The true incidence is unknown, but two recent studies have suggested that upwards of 50% of persons may have subclinical fluid accumulation in the lungs, consistent with occult edema which resolves spontaneously even though subjects remain at high altitude.[13,14]
PATHOPHYSIOLOGY
Exaggerated hypoxic pulmonary vasoconstriction and pulmonary hypertension
In contrast to systemic blood vessels which dilate in response to hypoxia, pulmonary blood vessels constrict. This constriction in non-homogenous, probably reflecting the distribution of smooth muscle in the walls of the arteries.[15] This response of the smooth muscle cells in the pulmonary vasculature begins within seconds and involves inhibition of voltage-dependent potassium channels, membrane depolarization and calcium entry through L-type calcium channels. Moreoever, hypoxia up-regulates transient receptor potential channels, leading to calcium entry through receptor and store-operated channels.[16]
Exaggerated hypoxic pulmonary vasoconstriction (HPV) can be attributed to increased susceptibility of the pulmonary vasculature-to sympathetic activity and/or high levels of endothelin-1. In addition, the endothelium mediated vasodilator response is blunted. This has been demonstrated by observing the response to acetylcholine in hypoxia measured by an impaired venous occlusion plethysmography in HAPE susceptible vs. non-susceptible controls.[17] Morevoever, upon acute exposure to hypoxia, exhaled nitric oxide concentrations and nitrite/nitrate in the BAL fluid tend to decrease in individuals prone to HAPE, whereas they increase in those resistant to the condition.[18–20] Also in susceptible individuals the prophylactic intake of tadalafil, a phosphodiesterase-5 inhibitor, prevents high altitude pulmonary hypertension and HAPE.[21]
Mechanisms accounting for increased capillary pressure and pulmonary capillary hypertension
There are at least two mechanisms responsible for the occurrence of pulmonary hypertension. The first is inhomogenous pulmonary vasoconstriction causing regional overperfusion of capillaries in areas of low arterial vasoconstriction.[11]
The second is hypoxic constriction occurring at the level of the pulmonary veins, increasing the resistance downstream of the region of fluid filtration.[22]
High permeability type of edema- ‘stress failure’ of the pulmonary capillaries
Broncho-alveolar lavage (BAL) performed in HAPE-susceptible adults within a day after ascent to 4559 m revealed elevated red blood cell counts and serum derived protein concentrations in BAL fluid.[18] However, the number of alveolar macrophages/ul and neutrophils/ul and the concentration of pro-inflammatory mediators interleukin-1(IL-1), TNF-α, IL-8, thromboxane, prostaglandin E2, and leukotriene B4(LTB4), was not increased. These results are consistent with studies done by West and colleagues[23] in rabbit lungs that showed that a rapid exposure of the pulmonary microcirculation to transvascular pressures of 40-60 cm H2O (30-44 mmHg) within 1 min is a hydraulic stress that exceeds the load bearing limits of the membrane collagen network and results in ruptures of the basement membrane and thus the alveolar-capillary barrier.[23,24] West had coined the structural engineering term ‘stress failure’ for this phenomenon.
In conclusion, the results of these studies indicate that HAPE is a hydrostatic type of pulmonary edema, the pathophysiological mechanism of which is excessive hypoxic pulmonary vasoconstriction of small arteries and veins, probably leading to over distension of the vessel wall, which opens the cellular junctions and possibly causes stress failure of the alveolo-capillary membrane.[11]
Reduced fluid clearance from the alveolar space
A further mechanism that may contribute to the pathophysiology of HAPE is a diminished capacity for alveolar fluid reabsorption. Removal of alveolar fluid is driven by the active reabsorption of Na+ that enters the cells via Na channels and Na-coupled transport (Na/X) and is extruded by Na+ K+ ATPases. Thus active Na reabsorption generates the osmotic gradient for the reabsorption of water. Results obtained in cultured alveolar epithelial cells and hypoxia-exposed rats shows that hypoxia:
- 1)Inhibits the activity and expression of various Na transporters, the most important being apical membrane epithelial Na channels and basolateral Na+ K+ ATPase
- 2)Decreases transepithelial Na transport
- 3)Decreases the reabsorption of fluid instilled into the lung.
Taken together, these findings suggest that impaired clearance of fluid filtered into the alveoli may be involved in the pathophysiology of HAPE.[25]
PREVENTION
Slow ascent is the most effective method of prevention, and one that is effective even in susceptible individuals. Apart from graded ascent and time for acclimatization, low sleeping altitudes, avoidance of alcohol and sleeping pills, and avoidance of exercise, are the key to preventing HAPE. Since exercise-induced circulatory changes may worsen or cause pulmonary edema, vigorous exercise should be avoided during the first days of altitude exposure by individuals with a history of HAPE.[1,11]
Research over the previous two decades has focused on mechanisms responsible for HAPE, and have centred primarily on the fact that accentuated pulmonary hypertension on ascent contributes to the development of HAPE. Bartsch et al. took this physiologic observation and tested the hypothesis that minimizing the pulmonary hypertension would prevent HAPE. They used a calcium channel blocker, nifedipine, in HAPE-s subjects on rapid ascent to 4559 m and essentially prevented HAPE. This study was considered an important link between clinical and physiological observations and therapeutic tools.[26] Hence, prophylaxis with nifedipine can be recommended in individuals with a history of unquestionable HAPE if slow ascent is not possible. 60 mg daily of a slow-release formulation should be given, starting with the ascent and ending on the third or fourth day after arrival at the final altitude, if the stay is to be prolonged, or after returning to an altitude below 3000 m or one to which the individual is acclimatized. It should be emphasized that nifedipine prevents HAPE, but is not effective for the treatment of acute mountain sickness.[11]
Acetazolamide is a carbonic anhydrase inhibitor and is effective in acute mountain sickness (AMS). It blunts hypoxic vasoconstriction in animals by its effect on calcium channels,[27,28] but further research is needed to determine its efficacy in HAPE.
Given the uncertainties with regard to the preventive effect of inhaled beta-2-receptor agonists, these drugs are not recommended for the prophylaxis of HAPE.[11]
According to preliminary data, glucocorticoids have been found to be effective in preventing HAPE in susceptible adults when taken one day prior to ascent and continued during ascent and stay at 4559 m. Glucocorticoids may be effective by stimulation of cGMP production in hypoxia, increasing in the activity of nitric oxide synthase and by increasing the activity of the epithelial Na+ K+ AT Pase pump. Although prophylaxis with dexamethasone for individuals susceptible to HAPE and AMS appears attractive, before general recommendation can be given further studies are needed to determine the minimal effective dose, its best route of administration (topical vs. systemic) and its safety profile in the setting of mountaineering.[11,12]
TREATMENT
Immediate improvement of oxygenation either by supplemental oxygen, hyperbaric treatment, or by rapid descent is the treatment of choice for HAPE. For the mountaineer in a remote area without medical care, descent has first priority, while the tourist with HAPE visiting a high altitude plateau in the Andes, Himalayas, or Rocky Mountains may stay at altitude if medical facilities are available. If it takes few days in a remote area to reach lower altitude, treatment with nifedipine is strongly recommended.[22] In mountaineers with HAPE at 4559 m, treatment with 20mg slow-release nifedipine taken every six hours led to a persistent relief of symptoms, improvement of gas exchange, and radiographic clearance over an observational period of 34 h. In this study, nifedipine therapy was not associated with hypotension.[29] To date, there are no clinical trials on the use of more selective pulmonary vasodilators such as sildenafil or other phosphodiesterase-5 inhibitors in this setting.
In an area where medical infrastructure and assistance are available, vasodilatory treatment is not strictly necessary because with bed-rest and supplemental oxygen for 24 to 48 hrs, relief of symptoms is achieved within hours and complete clinical recovery within several days while staying at the same altitude.[22]
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.